Lower Urinary Tract Innervation and its Development
Provided by Janet Keast, University of Melbourne and Michelle Southard-Smith, Vanderbilt University Medical Center
June 2021 (GUDMAP)
The urinary bladder is present in all mammals and serves to hold urine excreted by the kidneys. In humans, urination depends on appropriate innervation of the bladder so that urination can be performed at a socially acceptable time and place. In other mammals, urination is influenced by social hierarchies (scent marking) and holding urine to avoid predator detection. These processes require innervation of the muscular wall of the bladder (detrusor) to contract at the appropriate time and the urethra with its sphincter to maintain continence. This tutorial is focused on the nerves regulating the lower urinary tract (LUT) of rodents, providing specific examples from mice.
On this page:
- Neural structures of the lower urinary tract
- Motor innervation
- Source of motor innervation
- Sensory innervation
- Source of sensory innervation
- Structure of pelvic ganglia (PG) and associated nerves
- Development of pelvic ganglia
- Development of bladder innervation
- Resources
Neural structures of the lower urinary tract
Two classes of nerves (axons) are present in the LUT: motor and sensory. Electrical activity in motor axons changes the state of the cells within the LUT (e.g., muscle contraction, arterial constriction). Sensory axons detect changes within the LUT tissues (e.g., distension, pH) and electrically signal this information to the central nervous system (spinal cord and brain). Networks of neurons within the spinal cord and brain integrate the sensory signals from the LUT tissues with other relevant information (e.g., suitable time or location for voiding) to coordinate the appropriate changes in LUT tissues (e.g., to enable voiding or continence).
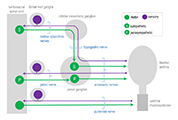
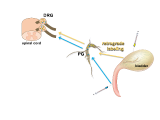
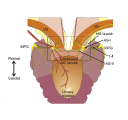
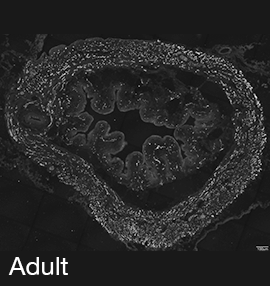
In this tutorial we will focus on the neural elements outside the central nervous system, i.e., in the periphery. These are illustrated in Figure 1.

Major components of the peripheral pathways innervating bladder, urethra and urethral rhabdosphincter. Arrows show the primary direction of electrical signaling. Most of the neural structures are bilateral, with only one side shown here. Sensory axons (purple) originate from neuronal cell bodies in dorsal root ganglia at several spinal levels. These axons travel with autonomic axons and via autonomic ganglia (green) to reach the appropriate tissues in the lower urinary tract. Most of the autonomic innervation of the bladder originates from neuronal cell bodies in the bilateral pelvic ganglia. A minority of the sympathetic innervation originates from the inferior mesenteric ganglia and sympathetic chain (not shown). For comparison, the innervation of the striated muscle urethral rhabdosphincter is shown. This is innervated by different populations of sensory neurons (purple) in dorsal root ganglia and motor neurons (green) originating in the spinal cord.
Motor innervation
Motor axons in the urinary bladder, urethra and their vasculature are part of the autonomic nervous system. Like the motor axons in other internal organs, they cannot be voluntary controlled. These axons are sometimes referred to as autonomic axons. Vascular and non-vascular smooth muscle have the highest density of autonomic innervation, although some autonomic axons are also located in the lamina propria, near the urothelium.
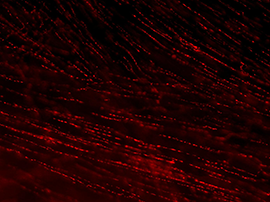
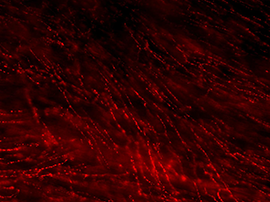
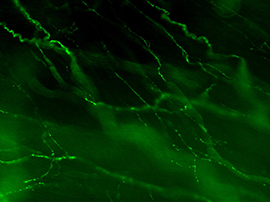
Each autonomic axon makes many synapses with the nearby tissue, indicated by their characteristic appearance [Figure 2]. Near their points of termination, they have numerous swellings (varicosities) that contain neurotransmitters. Motor axons are often visualized by immunohistochemically labeling transmitters or related proteins that are concentrated in these varicosities (e.g., enzymes required for transmitter synthesis or transmitter recycling at the synapse).
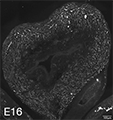
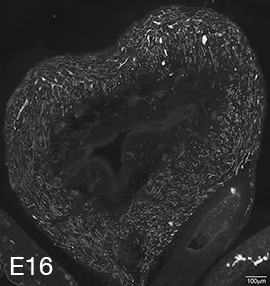
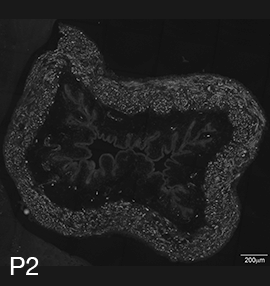
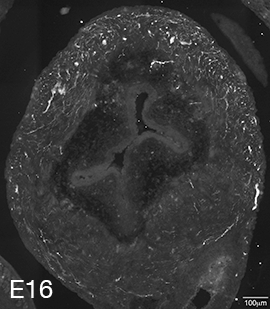
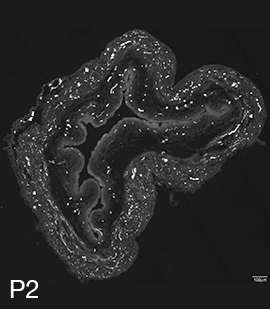
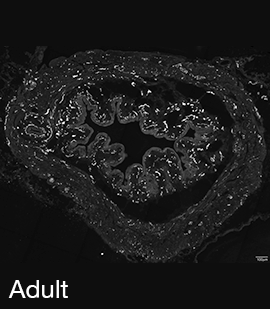
Figure 2A: Structure of cholinergic motor axons in the bladder (adult mouse).
Examples of cholinergic axons immunolabelled for vesicular acetylcholine transporter (VAChT; Slc18a3) in cryosections of mouse bladder at E16 (See GUDMAP RID N-FKKE), P2 (See GUDMAP RID N-FN0P) and adult (See GUDMAP RID N-FNFP).
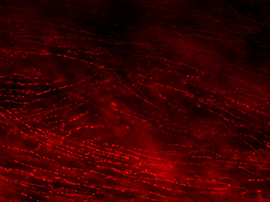
Figure 2B: Cholinergic axons in whole thickness adult mouse bladder immunolabelled for VAChT. Movie progresses through different focal planes in the muscle.
Figure 2C: Several individual focal planes from the movie above.
Autonomic axons in the LUT are classified as sympathetic or parasympathetic. This terminology is based on anatomy, not function. Specifically, the classification is determined by the location of the central nervous system neurons that determine the activity of the peripheral neural pathway of interest.
- Sympathetic nerve activity is determined by neurons in thoracolumbar spinal cord (sympathetic preganglionic neurons). In rodents, lumbar levels 1 and 2 (L1, L2) are the most important for LUT regulation, e.g., these are required for contraction of smooth muscle in the bladder neck and constriction of arterioles. Transmitters released by sympathetic nerves include norepinephrine (noradrenaline) and ATP.
- Parasympathetic nerve activity is determined by neurons in the brainstem or the sacral spinal cord (parasympathetic preganglionic neurons). In rodents, the most caudal lumbar segment, L6, and the first sacral segment, S1, are the most important for LUT regulation, e.g., these are required for bladder contraction during micturition. Transmitters released by parasympathetic axons include acetylcholine, nitric oxide and ATP.
Motor innervation of striated muscle in the LUT, the urethral rhabdosphincter (syn: external urethral sphincter) is quite different. This is under voluntary control so is innervated by somatic spinal motor neurons rather than autonomic circuits. Each motor axon branches to supply several sphincter striated muscle cells, releasing acetylcholine as the primary transmitter. There is a single, large transmitter release site at the end of each axon termination.
Source of motor innervation
In rodents, the major source of autonomic axons of the LUT is the pelvic ganglion (PG) (synonym: major pelvic ganglion, MPG). Smaller populations of neurons regulating the LUT are located in other sympathetic ganglia (inferior mesenteric ganglia, sympathetic chain) and small groups of neurons (each group usually <10 cells) located irregularly within the LUT tissues.
The PG is an unusual autonomic ganglion because it is a mixture of parasympathetic and sympathetic neurons, i.e., some of the neurons are controlled by L1-L2 spinal preganglionic neurons and some are instead controlled by L6-S1 spinal preganglionic neurons. In humans, the inferior hypogastric plexus is functionally homologous to the rodent PG.
The PG also contains autonomic neurons regulating reproductive organs and lower bowel. These are intermingled with the LUT neurons. There is currently no unique identifier of LUT neurons within the PG, so defining their specific molecular features requires alternative approaches such as retrograde tracing. This involves microinjecting a neural tracer into the organ of interest in vivo, under anesthetic; axons at the injection site take up the tracer and transport it to their cell bodies, which can then later be analyzed.
Motor innervation of the urethral rhabdosphincter originates from motor neurons in the ventral horn of the spinal cord, not the pelvic ganglion. These motor axons do not pass through the PG but travel to the sphincter directly from the spinal cord via the pudendal nerves.
Sensory innervation
Sensory axons in the urinary bladder, urethra and their vasculature are part of the visceral sensory system. Like the sensory axons in other organs, there are many aspects of their function which do not reach our conscious awareness. The muscle wall and the lamina propria near the urothelium have the highest density of sensory innervation, although some blood vessels are also supplied by sensory axons.
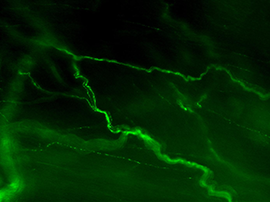
Sensory axons have a characteristic appearance within the LUT tissues [Figure 3]. These have various branching patterns at their terminals but generally do not have varicosities.
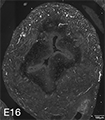
Figure 3A: Structure of peptidergic sensory axons in the bladder (adult mouse).
Examples of sensory axons immunolabelled for calcitonin gene-related peptide (CGRP; Calca) in cryosections of mouse bladder at E16 (See GUDMAP RID N-FM22), P2 (See GUDMAP RID N-FN1T) and adult (See GUDMAP RID N-FNF2).
Figure 3B: Sensory axons in whole thickness adult mouse bladder immunolabelled for CGRP. Movie progresses through different focal planes in the muscle.
Figure 3C: Several individual focal planes from the movie above.
Two major classes of LUT sensory axon are nociceptors (expressing ion channels that transduce tissue-damaging stimuli, e.g., Trpv1, Trpa1) and mechanoreceptors (responding to local mechanical changes such as distension). Nociceptors have thin, unmyelinated axons (C-fibers) whereas mechanoreceptors have thicker axons surrounded by myelin (A-delta fibers). Both types of axons are present in the bladder, urethra and urethral rhabdosphincter, but sensory axons in the bladder have been characterized in the most detail.
Source of sensory innervation
Sensory axons in the bladder originate from neuronal cell bodies in lumbar or sacral dorsal root ganglia (DRG). These ganglia lie close to the spinal cord, so their axons travel a long distance to reach the LUT tissues.
The lumbar (L1, L2 axial levels) and sacral (L6, S1 axial levels) DRG also contain sensory neurons that have no role in LUT function (e.g., innervate other pelvic organs, or somatic tissues such as the skin or muscle of the hindlimb). In any DRG, LUT neurons are in the minority (<5% and ~10% of L1/L2 and L6-S1 DRG neurons, respectively).
There is currently no unique identifier of LUT neurons within the DRG, so defining their specific molecular features requires alternative approaches such as retrograde tracing. This involves microinjecting a neural tracer into the organ of interest; axons at the injection site take up the tracer and transport it to their cell bodies in DRG, which can then be analyzed. This method will also label any motor/autonomic neurons innervating the injection site.
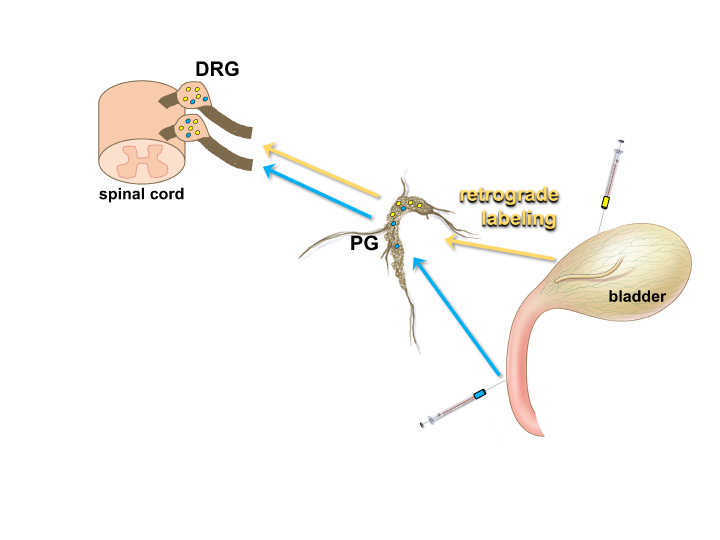
Figure 4: Using retrograde tracers to identify neurons that innervate specific structures in the LUT.
The location of neuronal cell bodies that provide innervation of a particular organ can be determined by injection of the organ wall with an appropriate tracer (e.g. FluoroGold (yellow) or Fast Blue (blue)). These dyes are taken up by nerve terminals in the target organ and transported up axonal processes to the neuronal cell body, such as those located within the pelvic ganglia (PG) or dorsal root ganglia (DRG). This method allows the specific position of neurons that provide innervation of discrete structures such as the detrusor of the bladder or the urethra to be determined by fluorescent microscopy viewing of sections through the DRG or PG. (Illustration of bladder by Alice McKinney.)
Structure of pelvic ganglia (PG) and associated nerves
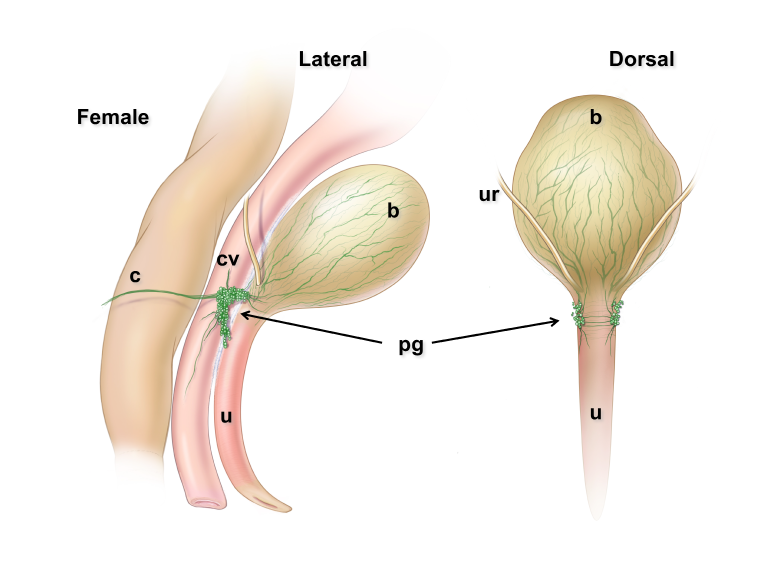
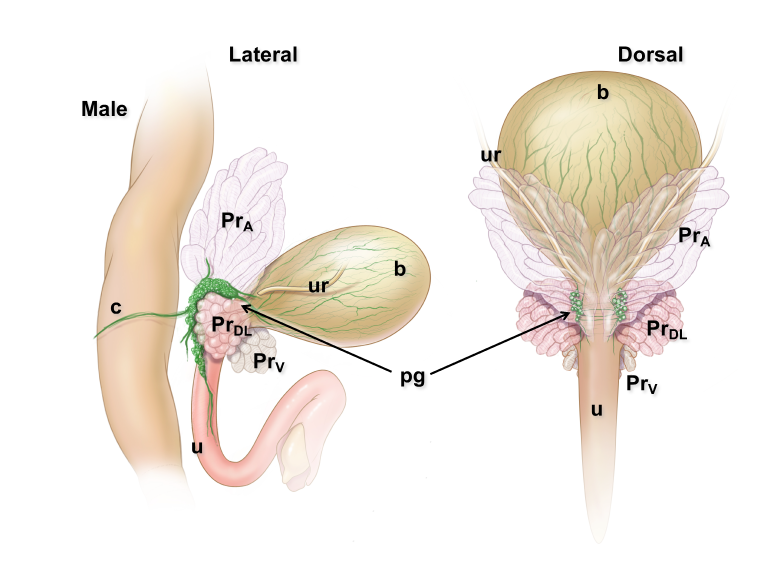
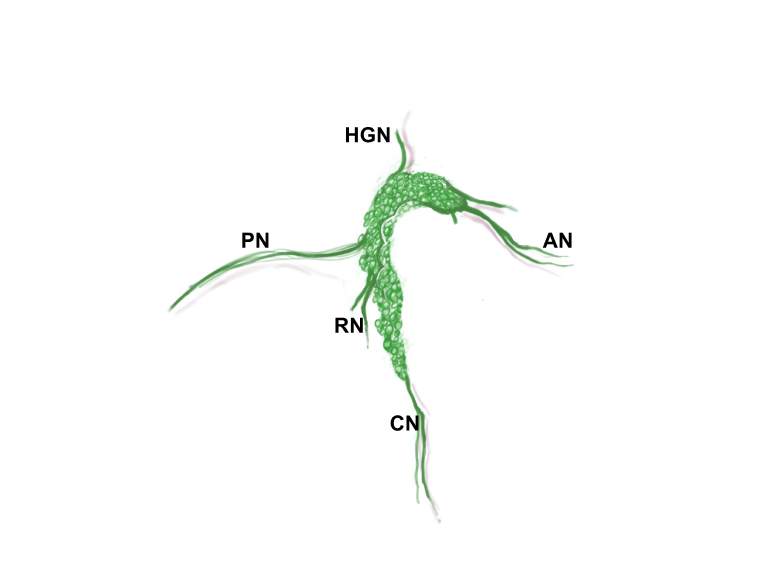
Pelvic ganglia (PG) are paired ganglia that innervate the lower urinary and intestinal tracts, and reproductive organs. A realistic illustration of the PG structure and location in adult male and female mice is shown below [Figure 5]. As shown in this diagram, several major nerve tracts are attached to each PG, most of which are “mixed nerves” that contain both sensory and motor axons. These have characteristic locations and composition. There are also several smaller ganglia that are contiguous with the pelvic ganglia and its nerves, sometimes referred to as accessory ganglia. In the mouse these accessory ganglia are prominently clustered at the bladder neck near the ureter insertion sites.
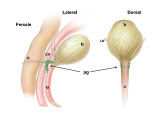
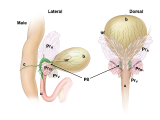
Figure 5: Location and features of pelvic ganglia in adult mice.
Schematic diagrams of the lower pelvic organs of adult female mice (left) illustrate the position of the paired pelvic ganglia just lateral to the uterine cervix and slightly dorsal to the bladder neck. At right, schematic diagrams of lower pelvic organs for adult male mice illustrate the location of the paired pelvic ganglia positioned lateral to the anterior prostate lobes and dorsal to the prostatic urethra/bladder neck.
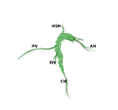
Zoomed/cropped lateral view from male and female, with labelled pelvic ganglia and nerves. b, bladder; cv, uterine cervix; pg, pelvic ganglia; Pr, prostate, (A) anterior, (V) ventral, (DL) dorsolateral lobes; c, colon; u, urethra; AN, accessory nerves; CN, cavernous nerve; HGN, hypogastric nerve; PN, pelvic nerve; RN, rectal nerves. (Illustrations by Alice McKinney.)
Pelvic nerve contains axons from:
- L6 and S1 spinal autonomic neurons (parasympathetic preganglionic neurons): these synapse on and activate parasympathetic PG neurons.
- L6 and S1 dorsal root ganglia: these sensory axons travel through the PG to reach their relevant pelvic organ. These axons comprise the majority of bladder sensory nerves and are required for micturition.
- Sympathetic chain ganglia: these sympathetic axons travel through the PG to innervate vasculature in the pelvic organs.
Hypogastric nerve contains axons from:
- L1 and L2 spinal autonomic neurons (sympathetic preganglionic neurons): these synapse on and activate sympathetic PG neurons.
- L1 and L2 dorsal root ganglia: these sensory axons travel through the PG to reach their relevant pelvic organ. These axons comprise a minority of bladder sensory nerves. Their function is not well understood.
Accessory nerves and rectal nerves contain axons from:
- PG neurons that project to the LUT or reproductive organs (accessory nerves) or lower bowel (rectal nerves).
- DRG neurons that project via the PG to the relevant pelvic organs.
Cavernous (M) or clitoral (F) nerve contains axons from:
- PG neurons that project to erectile tissue of the penis/clitoris.
In addition to the PG, there are also smaller ganglia that are located near the bladder neck. There are usually two to four of these accessory ganglia, which in male rodents are found bilaterally on the surface of the prostate between the ureter and the vas deferens [Figure 6]. These ganglia are more variable in size and shape and are connected to the PG by short, fine nerve bundles. Contralateral nerve fibers extending from these accessory ganglia extend behind the most anterior aspect of the urethra.
Figure 6: Diagram of male pelvic circuitry illustrates location of pelvic ganglia (major pelvic ganglia) relative to accessory ganglia.
Modified from Arellano et al., 2019 (https://doi.org/10.1016/j.autneu.2018.12.005). Ventral view of the urogenital tract. Major pelvic ganglia (MPG) are connected to accessory ganglia (AG) by ipsilateral connections and commissural nerves (1-4). Vas deferens (VD); Hypogastric nerve branch (HG).
Development of pelvic ganglia
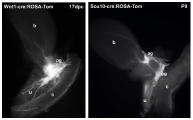
Pelvic ganglia (PG) originate from neural crest progenitors that migrate from the dorsal aspect of the neural tube towards the urogenital sinus early in embryonic development. This process commences at approximately 9.75 days post coitus (dpc) in the mouse. These progenitors have been visualized by tracking expression of migratory neural crest genes, like Sox10, or lineage labeling by cre-LoxP strategies using Wnt1-cre:ROSA reporters. Masses of these progenitors migrate around the fetal hindgut and approach the urogenital sinus where they aggregate into prominent clusters at either side of the bladder neck. Between 13.5-14.5dpc in the mouse, neural processes extend from the pelvic ganglia out into the bladder body towards the bladder dome [Figure 7].